Stevens-Johnson syndrome and toxic epidermal necrolysis
(Redirected from Toxic Epidermal Necrolysis)
Background
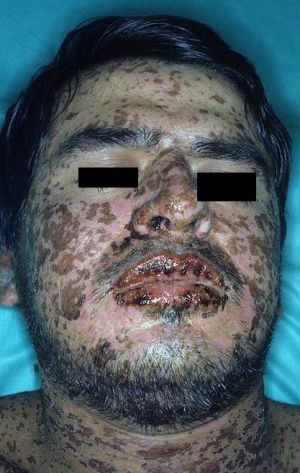
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are a spectrum of disease which ultimately results in blistering and peeling of the skin
- Mucous membranes can also be involved
- SJS and TEN exist on a spectrum of disease
- SJS involves <10% of the skin body surface area
- TEN involves >30% of the skin body surface area
- SJS and TEN are considered true dermatologic emergencies
Causes
- Drugs
- The most common cause overall[1]
- Many have been linked. Common offensive agents include: sulfa, quinolones, PCN, ASA, acetaminophen, carbamazepine, NSAIDs, phenytoin, corticosteroids, immunizations
- High dose or rapid loading of allopurinol[2], lamotrigine[3]
- Malignancy - lymphoma, brain tumor treated with radiotherapy and antiepileptics[4]
- Idiopathic
- Immunosuppression - HIV [5]
- Infectious - Mycoplasma pneumoniae[6]
- Autoimmune - SLE[7]
Clinical Features
- Often have prodrome (fever, URI symptoms, headache, malaise)
- Macular rash
- +/- Target lesions
- Usually starts centrally, spreads peripherally, and may become confluent
- May be painful
- May have +Nikolsky sign (denude when touched)
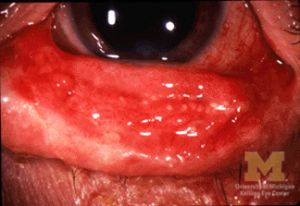
- Mucous membranes can be severely affected
- Eye involvement can be severe
- In severe cases, respiratory tract and GI involvement may occur
Differential Diagnosis
- Erythema Multiforme
- Staphylococcal scalded skin syndrome
- Erythroderma
- Toxic Shock Syndrome
- Drug rash
- Acute generalized exanthematous pustulosis
- DRESS syndrome
Oral rashes and lesions
- Angioedema
- Aphthous stomatitis
- Herpes gingivostomatitis
- Herpes labialis
- Measles (Koplik's spots)
- Perioral dermatitis
- Oral thrush
- Steven Johnson syndrome
- Streptococcal pharyngitis
- Tongue diagnoses
- Vincent's angina
Evaluation
Work-Up
- CBC
- CMP
- ESR
- CXR
- Examine eyes/mucosal surfaces
Evaluation
- Clinical diagnosis
Table of Severe Drug Rashes
| Charateristic | DRESS | SJS/TEN | AGEP | Erythroderma |
| Image |  |
 |
 |

|
| Onset of eruption | 2-6 weeks | 1-3 weeks | 48 hours | 1-3 weeks |
| Duration of eruption (weeks) | Several | 1-3 | <1 | Several |
| Fever | +++ | +++ | +++ | +++ |
| Mucocutaneous features | Facial edema, morbilliform eruption, pustules, exfoliative dermattiis, tense bullae, possible target lesions | Bullae, atypical target lesions, mucocutaneous erosions | Facial edema, pustules, tense bullae, possible target lesions, possibl emucosal involvement | Erythematous plaques and edema affecting >90% of total skin surface with or without diffuse exfoliation |
| Lymph node enlargement | +++ | - | + | + |
| Neutrophils | Elevated | Decreased | Very elevated | Elevated |
| Eosinophils | Very elevated | No change | Elevated | Elevated |
| Atypical lymphocytes | + | - | - | + |
| Hepatitis | +++ | ++ | ++ | - |
| Other organ involvement | Interstitial nephritis, pneumonitis, myocarditis, and thydoiditis | Tubular nephritis and tracheobronical necrosis | Possible | Possible |
| Histological pattern of skin | Perivascular lymphocytcic infiltrate | Epidermal necrosis | Subcorneal pustules | Nonspecific, unless reflecting Sezary syndrome or other lymphoma |
| Lymph node histology | Lymphoid hyperplasia | - | - | No, unless reflecting Sezary syndrome or other malignancy |
| Mortality (%) | 10 | 5-35 | 5 | 5-15 |
Management
- Removal of inciting cause if identified
- Fluid replacement - treat shock with IV fluids according to burn protocols
- Infection control
- Wound care
- Use of IVIG, plasmapheresis, and corticosteroids are controversial but may be beneficial
- Evidence that Etanercept (TNF-alpha antagonist) may decrease time to skin healing and mortality compared to IV prednisolone [8]
Disposition
- Admit to burn unit or ICU
Prognosis
Validated with SCORTEN mortality assessment:
One point for each of the following assessed within 1st 24 hours of admission:
- Age >/= 40 years (OR 2.7)
- Heart Rate >/= 120 beats per minute (OR 2.7)
- Cancer/Hematologic malignancy (OR 4.4)
- Body surface area on day 1; >10% (OR2.9)
- Serum urea level (BUN) >28mg/dL (>10mmol/L) (OR 2.5)
- Serum bicarbonate <20mmol/L (OR 4.3)
- Serum glucose > 252mg/dL (>14mmol/L) (OR5.3)
Predicted mortality based on above total:
| Score | Mortality |
| 0-1 | 3.2% |
| 2 | 12.1% |
| 3 | 35.3% |
| 4 | 58.3% |
| 5+ | 90.0% |
See Also
References
- ↑ Mockenhaupt M (2011). "The current understanding of Stevens–Johnson syndrome and toxic epidermal necrolysis". Expert Review of Clinical Immunology 7 (6): 803–15. doi:10.1586/eci.11.66. PMID 22014021
- ↑ Halevy S, Ghislain PD, Mockenhaupt M, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008 Jan. 58(1):25-32. [Medline]
- ↑ Schlienger RG, Shapiro LE, Shear NH. Lamotrigine-induced severe cutaneous adverse reactions. Epilepsia. 1998. 39 Suppl 7:S22-6. [Medline]
- ↑ Medscape: Stevens-Johnson Syndrome
- ↑ Rotunda A, Hirsch RJ, Scheinfeld N, Weinberg JM. Severe cutaneous reactions associated with the use of human immunodeficiency virus medications. Acta Derm Venereol. 2003. 83(1):1-9. [Medline]
- ↑ Kunimi Y, Hirata Y, Aihara M, Yamane Y, Ikezawa Z. Statistical analysis of Stevens-Johnson syndrome caused by Mycoplasma pneumonia infection in Japan. Allergol Int. 2011;60(4):525-532. doi:10.2332/allergolint.11-OA-0309
- ↑ Horne NS, Narayan AR, Young RM, Frieri M. Toxic epidermal necrolysis in systemic lupus erythematosus. Autoimmun Rev. 2006 Feb. 5(2):160-4. [Medline]
- ↑ Wang, C.-W., Yang, L.-Y., Chen, C.-B., Ho, H.-C., Hung, S.-I., Yang, C.-H., … and the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. (2018). Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. The Journal of Clinical Investigation, 128(3), 985–996.