Marfan syndrome: Difference between revisions
No edit summary |
|||
| Line 108: | Line 108: | ||
[[Category:Misc/General]] | [[Category:Misc/General]] | ||
===Disposition=== | ===Disposition=== | ||
Revision as of 16:11, 27 August 2025
BACKGROUND [1]
- Marfan syndrome (MFS) is a heritable connective tissue disorder with multi-system involvement
- First characterized as a syndrome by French pediatrician Antoine Marfan in 1896
- Clinical features vary along a spectrum typical of autosomal-dominant disorders
- Autosomal-dominant mutation in FBN1 gene (encodes collagen matrix protein fibrillin-1) on chromosome 15
- This results in cystic medial degeneration of the aortic tunica media (leading to increased risk of aortic aneurysm / dissection)
- This also interferes with elastin deposition during extracellular matrix formation implicates in the elasticity of multiple tissue types
- Majority of cases (75%) are familial / inherited vs. minority (25%) are de novo mutations
- Estimated prevalence of 1/5000 individuals worldwide (equal between men and women)
- Life expectancy for those diagnosed and treated is now close to that of non-MFS population (previously expected increase in patient mortality by third and fourth decades of life)
CLINICAL FEATURES & DIAGNOSIC CRITERIA [2]
Revised Ghent Nosology (2010)
In the absence of family history:
- Aortic Root Dilatation Z Score > 2 and Ectopia Lentis
- Aortic Root Dilatation Z Score > 2 and FBN1 Mutation
- Aortic Root Dilatation Z Score > 2 and Systemic Score > 7 points
- Ectopia Lentis and FBN1 Mutation (associated with aortic root dilatation)
In the presence of family history:
- Ectopia Lentis and Family History of Marfan Syndrome (MFS)
- Systemic Score > 7 points and Family History of MFS
- Aortic Root Dilatation Z Score > 2 (above 20 years old), > 3 (below 20 years old) and Family History of MFS
Clinical Features (not all may be present) (Figure 1)
- Tall stature, long extremities
- Reduce upper-to-lower segment ratio, increased arm span-to-height ratio
- Arachnodactyly (“wrist sign, thumb sign), reduced elbow extension
- Scoliosis or thoracolumbar kyphosis
- Pectus excavatum or carinatum
- Ligamentous laxity, hyperextensibility
- Protrusio acetabuli
- Hindfoot deformity, plain flat foot
- Ectopia lentis
- Myopia (often severe); retinal detachment
- Lumbrosacral dural ectasia
- Dolichocephaly, downward slanting palpebral fissures, enophthalmos, retrognathia, malar hypoplasia, high arched palate
- Skin striae
Increased Risk of the Following [3]
- Acute Aortic Syndrome (AAS)
- Thoracic aortic aneurysm
- Stanford Types A and B Aortic Dissection
- Intramural Hemotoma (IMH)
- Mitral valve prolapse (present in up to 60%) and mitral regurgitation
- Spontaneous pneumothorax (associated with bullae, 4-11%)
- Subarachnoid hemorrhage (SAH)[4]
- Controversial link between Marfan Syndrome and intracranial aneurysms (more clearly associated with vEDS and LDS)
- Ocular Lens dislocation, retinal detachment
- Spinal conditions (scoliosis; lumbosacral disease; dural ectasia)
- Musculoskeletal injuries due to joint laxity (laxity in 85% of children, 56% of adults)
- Complications during pregnancy (risk of aortic dissection)
- Type A dissection risk increases with aortic dilation; Type B risk poorly understood. (aortic dissection in up to 4.5%, primarily peripartum)
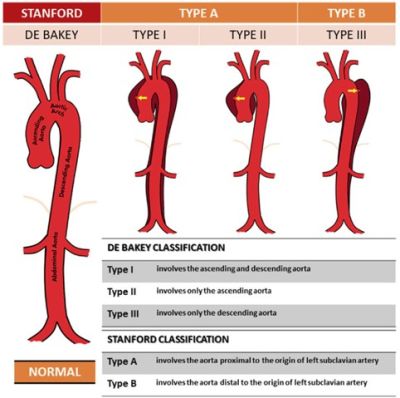
Figure 2. Classification of Aortic Dissection using Stanford and DeBakey systems. Source: ResearchGate
DIFFERENTIAL DIAGNOSIS[2]
- Classic vs. Kyphoscoliotic vs. Vascular Ehlers-Danlos Syndrome (vEDS)
- Loeys-Dietz Syndrome (LDS)
- Familial Thoracic Aortic Aneurysm and Dissection (FTAAD)
- Familial Ectopia Lentis Syndrome
- MASS Phenotype (Myopia, Mitral Valve Prolapse, Aortic Root Dilatation, Aortic Aneurysm Syndrome, Striae, Skeletal Findings)
- Shprintzen-Goldberg Syndrome
- Beals Syndrome
- Stickler Syndrome
- Non-Specific Connective Tissue Disorder
MANAGEMENT
Medications
- Beta-Blocker (BB) and/or Angiotensin II Type 1 Receptor Blocker (ARB) Therapy
- Beta-blockade lowers blood pressure / heart rate and reduces aortic wall shearing forces to reduce the rate of aortic aneurysm growth and risk of dissection
- Inhibition of ATII Type 1 Receptor-mediated TGF-beta signaling may reduce elastic tissue degeneration based upon mouse models.[4]
- Other Pharmacologic Considerations
- Avoidance of fluoroquinolones (FQs) due to matrix metalloproteinase (MMP) activation and increased risk of aortic aneurysm/dissection
- Chen S-W et al. (2023) did not show a significant increase in aortic aneurysm/dissection (OR 1.000) in MFS and other high-risk patients after FQ administration[5]
- Gopalakrishnan et al. (2020) showed a significant increase in aortic aneurysm/dissection in patients receiving FQs for pneumonia (HR 2.57, p<0.01%), but not UTIs (HR 0.99, p<0.01%). This suggests intrathoracic inflammation from the primary disease pathology, not FQs themselves, increase the risk of aneurysm or dissection[6]
- Antibiotic prophylaxis is no longer recommended for MFS patients before dental procedures, except in those with artificial heart valves
- Avoidance of fluoroquinolones (FQs) due to matrix metalloproteinase (MMP) activation and increased risk of aortic aneurysm/dissection
Lifestyle Modifications[7]
- Regular blood pressure measurement, lifestyle modification, and titration of antihypertensive therapies with goal BP < 120/80 mmHg
- Avoidance of high-intensity, high-impact sports and intensive isometric activities / weightlifting
- Low- to moderate-intensity aerobic activities are encouraged after discussion with a cardiologist or specialist
- Avoidance of Valsalva and other maneuvers that increase intrathoracic or abdominal pressure (risk of acute dissection)
- Avoidance/cessation of tobacco, smoking, vaping, or stimulant abuse (e.g., cocaine, methamphetamines)
- Psychiatric evaluation, resources, and support groups (e.g., Marfan Foundation, GenTAC) as appropriate to help address psychosocial sequelae (e.g., body dysmorphia, anxiety, depression, PTSD, suicidal ideation)
Emergency Management[8][9]
- Evidence-based management of acute aortic dissection:
- IV medications to reduce blood pressure (< 100–120 mmHg) and heart rate (< 60 bpm) (e.g., esmolol, labetalol, nitroprusside)
- Emergent vascular, cardiothoracic, or other surgical consultations
- Definitive management per current guidelines based on Stanford Type A vs. B dissection, location, extent, and branch involvement
- Standard-of-care treatment for other associated pathologies
Disposition
- Disposition depends entirely upon the chief complaint, clinical gestalt, and objective findings from physical exam and workup.
See Also
External Links
- Marfan Foundation
- Marfan Foundation (Resources for Physicians)
- GenTAC Alliance
- International Registry of Acute Aortic Dissection (IRAD)
References
- ↑ Milewicz DM, Braverman AC, De Backer J, et al. Marfan syndrome. Nat Rev Dis Primers. 2021;7(1):64. doi:10.1038/s41572-021-00298-7.
- ↑ 2.0 2.1 The Marfan Foundation. (2025, May 21). The Marfan Foundation | Know the Signs | Fight for Victory. Marfan Foundation. https://marfan.org/
- ↑ Dean, J. Marfan syndrome: clinical diagnosis and management. Eur J Hum Genet. 2007;15:724-733. doi.org/10.1038/sj.ejhg.5201851
- ↑ Reference 7
- ↑ Reference 8
- ↑ Reference 9
- ↑ Reference 10
- ↑ Reference 11
- ↑ Reference 12
References
- Kim JH, Woo Kim J, Song S-W, et al. Intracranial aneurysms are associated with Marfan Syndrome: Single cohort retrospective study in 118 patients using brain imaging. Stroke. 2020;52(1):331-334. doi.org/10.1161/STROKEAHA.120.032107


